Specific Heat Capacity and Specific Latent Heat
Specific Heat Capacity and Specific Latent Heat: Overview
This topic covers concepts, such as, Specific Heat Capacity, Heat, Determination of Specific Heat of a Solid & Determination of Specific Latent Heat of Vaporisation of Water etc.
Important Questions on Specific Heat Capacity and Specific Latent Heat
When a substance melts, the required energy is called a specific latent heat of vaporisation.
An unknown solid of mass of solid at is allowed to sink in of water at . Calculate the specific heat of the unknown solid in the units of if the final temperature of water becomes . Specific heat of water is and density of water is .
water at is mixed with another liquid of mass at . After mixing, they attain a mutual temperature of . If the specific heat capacity of water is , determine the specific heat capacity of the other liquid in the same unit.
Consider an experiment in which a beaker containing water is heated using an electrical heater. A wattmeter determines the rate at which energy is supplied to the heater. The beaker is insulated to minimise energy loss, and it stands on a balance. A thermometer is included to ensure that the temperature of the water remains at .
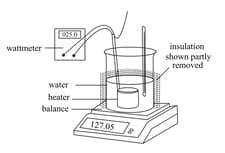
The water is heated at steady rate and its mass recorded at equal intervals of time. It decreases as it boils.
What is the relation between the calculated latent heat in the experiment and true latent heat?
Explain a method to determine the specific latent heat of vaporisation of water.
The SI unit of specific heat is .
Amongst object A and B, if the specific heat of object A is less than of object B, then
The specific heat capacity of any substance is the amount of heat required to raise the temperature of one unit substance by hundred degree.
The amount of heat required to increase the temperature of one kilogram of a material, by is called latent heat.
A cube of lead of at is supplied with a heat. Find the final temperature (in degree Celsius) of the lead cube. (Take, specific heat of lead is )
Calculate the heat energy (in joules) to raise the temperature of of water from to . (Take specific heat of water, )
Specific heat capacity of a substance depends on the mass of the substance.
When of brass at is dropped into of water at the resulting temperature is . What must have been the specific heat of brass ?
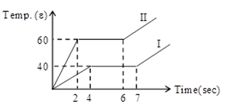
Two solid bodies of equal masses are heated at a uniform rate under identical conditions. The change in temperature in the two cases is shown graphically. What is the ratio of specific heat in solid state
Specific heat of a solid depends on which of the following factors?
of water at is divided into two parts so that one part of mass when converted into ice at would release enough heat to vapourise the other part, then is equal to
The value of and for a gas are and . The vapour density of gas is . Its atomic mass will be
A drilling machine of power is used to drill a bore in a small aluminium block of mass . If of power is used up in heating the machine itself or lost to the surroundings then how much is the rise in temperature of the block in minutes?
(Given: specific heat of aluminium )
The specific heat of a substance at temperature is Calculate the amount of heat required to raise the temperature of of the substance from to
Three liquids with masses are thoroughly mixed. If their specific heats are and their temperatures respectively, then the temperature of the mixture is